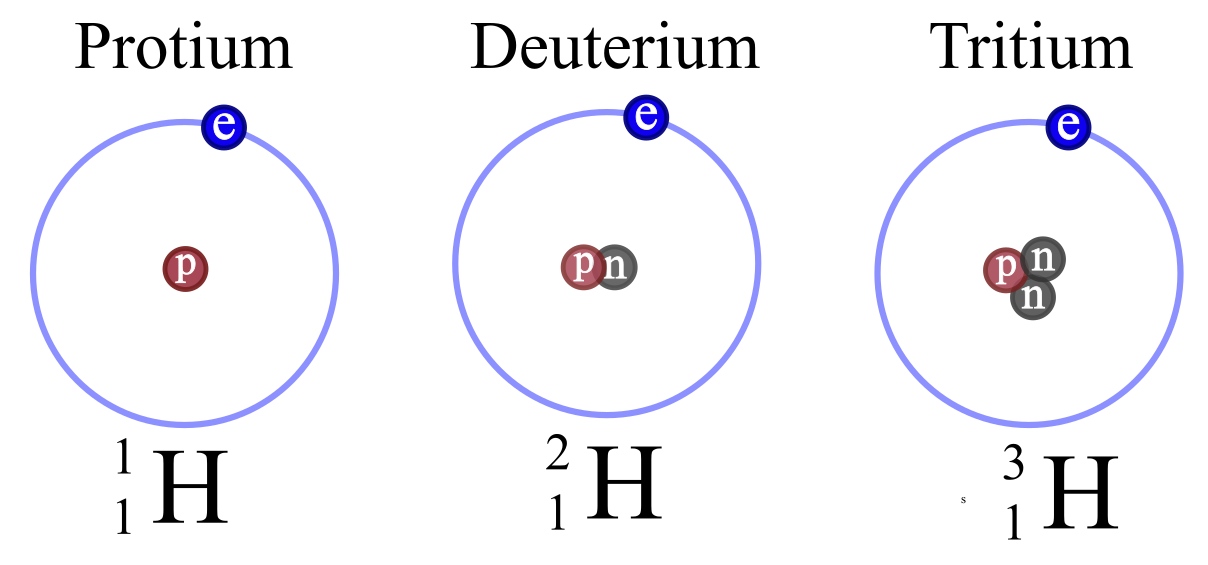
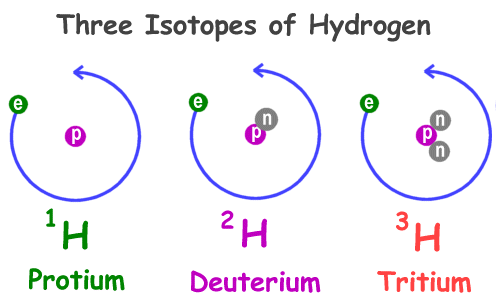
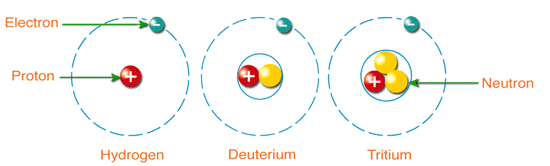
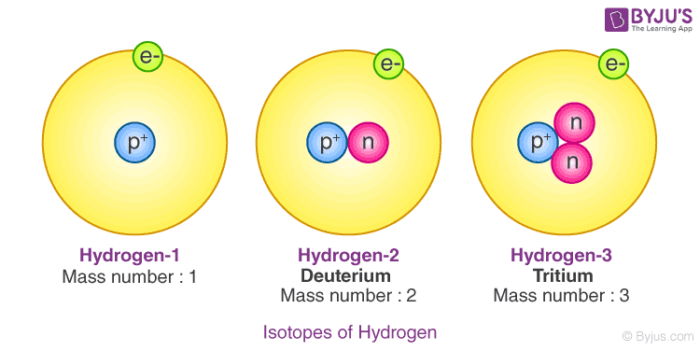
Discovering the Essential Universe 5th. Since all 3 isotopes of Hydrogen have different mass numbers they have different physical properties Lets compare the physical properties of the isotopes.
From Simple English Wikipedia the free encyclopedia The three most stable isotopes of hydrogen Hydrogen has three main isotopes.

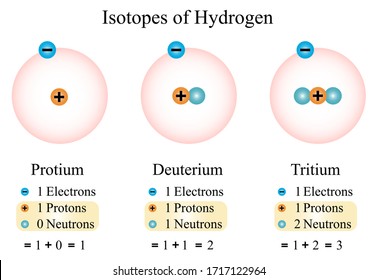
Three isotopes of hydrogen. There are three primary isotopes of hydrogen. Three isotopes of hydrogen have been found by scientists in nature and each of them can combine with oxygen. Protium Deuterium and Tritium.
How are isotopes of the sa 0048. Naturally occurring tritium is extremely rare on Earth. Tritium has the highest melting and boiling point out of the 3 isotopes.
What do all isotopes. There are three isotopes for hydrogen with mass numbers 1 2 and 3 each possessing an atomic number of one. The nucleus of this isotope consists of only a single proton atomic number mass number 1 and its mass is.
Protium or hydrogen-1 deuterium or hydrogen-2 and tritium or hydrogen-3. With a ring around it a sphere around the nucleus a four-leave clover two lobes apposing each other Examine the three isotopes of potassium K below. Protium Deuterium Tritium 27 Sep 2013 3 Comments Elements are the building blocks of a chemists world.
The electrical charge on all three is the same and the chemical properties are identical. Protium or ordinary hydrogen. Gallium is a metal with a wide variety of uses.
Isotopes are atoms of the same element having same atomic number but different. Hydrogen has three main isotopes. You must be signed in to discuss.
The nucleus of hydrogen consists of one solitary proton deuterium has one neutron as well as the proton and tritium contains one proton and two neutrons. Three isotopes of hydrogen. Protium deuterium and tritium.
1 H protium 2 H deuterium and. 1 H is the most common hydrogen isotope with an abundance of more than 9998. Isotopes- Atoms of the same element having same atomic number but different mass number are called isotopes.
Light and Telescopes Discussion. It has a natural. These isotopes form naturally in nature.
Summary of isotopes of hydrogen. Protium deuterium and tritium waters. Hydrogen in particular has three isotopes.
Isotopes of Hydrogen Properties of Isotopes of Hydrogen. The nucleus of tritium sometimes called a triton contains one proton and two neutrons whereas the nucleus of the common isotope hydrogen-1 protium contains just one proton and that of hydrogen-2 deuterium contains one proton and one neutron. Radioactive isotopes of gallium are.
If playback doesnt begin shortly try restarting your device. Hydrogen has three naturally occurring isotopes denoted 1 H 2 H and 3 H. Protium 1 H deuterium 2 H and tritium 3 H.
There may also be mixed waters containing say an atom of protium and an atom of. Scientists have created four other hydrogen isotopes but these isotopes are very unstable and do not exist naturally. Hydrogen has three naturally occurring isotopes.
H 2O D 2O and T 2O respectively. The Three Isotopes of Hydrogen. Protium and deuterium are stable.
Tritium is also the densest out of the 3 isotopes. These isotopes form naturally in nature. Hence one may speak of three kinds of water.
How are the three isotopes of hydrogen different from each other. The hydrogen atom has two isotopes. The structure of the three isotopes of hydrogen are.
Tritium is radioactive and has a half-life of about 12 years. The main isotopes of hydrogen. Other highly unstable nuclei 4 H to 7 H have been synthesized in the laboratory but are not observed in nature.
How do isotopes of a given 0013. Some of its applications include computer memory chips light emitting diodes and lasers. Unlike any of the other isotopes they have special names.
 Isotopes Of Hydrogen Plutonium Deuterium Tritium With Examples Videos
Isotopes Of Hydrogen Plutonium Deuterium Tritium With Examples Videos
 Isotopes Of Hydrogen Teaching Chemistry Chemistry Math Help
Isotopes Of Hydrogen Teaching Chemistry Chemistry Math Help
 Isotope Simple English Wikipedia The Free Encyclopedia
Isotope Simple English Wikipedia The Free Encyclopedia
 Hydrogen Isotopes High Res Stock Images Shutterstock
Hydrogen Isotopes High Res Stock Images Shutterstock
 The Structure Of The Three Isotopes Of Hydrogen
The Structure Of The Three Isotopes Of Hydrogen
Isotopes Of Hydrogen Wikipedia
 Nasa Giss Science Briefs Isotopically Speaking Tracing The Water Cycle
Nasa Giss Science Briefs Isotopically Speaking Tracing The Water Cycle