Specific Heats and Heat of Fusion of Iodine. It is naturally emitted by rice plantations in small amounts.
 Nick Connor Autor Material Properties Page 80 Of 214
Nick Connor Autor Material Properties Page 80 Of 214
Specific heat or.
Specific heat of iodine. K at room temperature and decreases monotonically to 16 mWcm. 16 - 23 141 - 21. Thermal Conductivity of Common Metals and Alloys.
Palm oil 506 - 55. Iodine value g100 g. The specific heats of iodine vapour and solid are 0031 and 0055 calg respectively.
As a gas it is violet and intensely irritating to. The thermal conductivity of pure CHx is about 75 mWcm. Q 2 kJkg K 10 kg 30 o C 600 kJ.
Palm stearin Palm oil neutralized. The specific heat dependence with temperature is roughly linear from about 05 Jcm3. Specific heat of Iodine is 0214 Jg K.
Specific heat of Iodine is 0214 Jg K. Given that the specific heats of iodine vapour and solid are 0031and 005calgrespectively. 0 3 1 and 0.
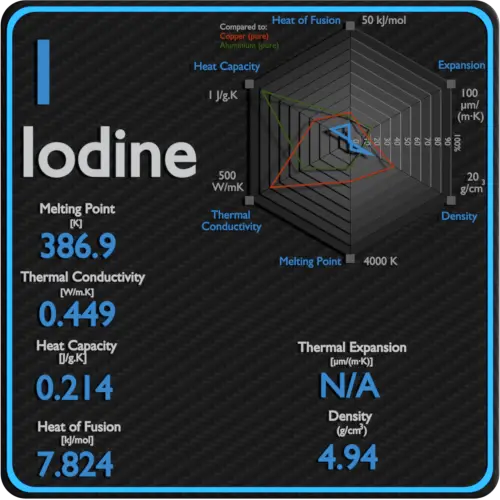
In succession to the previous study on the low temperature specific heat of ICl-graphite intercalation compounds below 5 K a theoretical analysis has been made on the data obtained in the higher temperature range up to 300 K using a semi-continuum model similar to that originally proposed by Komatsu and Nagamiya for graphite. If 10 kg of oak is heated from 20 o C to 50 o C - a temperature difference 30 o C K the heat required can be calculated as. Latent Heat of Vaporization of Iodine is 20752 kJmol.
50 - 55 56. It is a dense colorless volatile liquid. 180 - 205 Palm kernel oil.
Once used as an antiseptic but no longer due to its poisonous nature. Palm olein neutralized 56. K at 25 K.
8 Zeilen Specific heat of Iodine is 0214 Jg K. Specific Heat Capacity of Metals Table Chart. Molar heat capacity of iodine solid 005127635.
The specific heats of iodine vapours and solid are 0. Oil Fat Olive oil. The heaviest of the stable halogens it exists as a lustrous purple-black non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 degrees Celsius and boils to a violet gas at 184 degrees Celsius.
Q heat required kJ c p specific heat kJkg K kJkg o C dt temperature difference K o C Example - Required Heat to increase the Temperature in a Piece of Oak. Palm olein crude 56. The specific heat of the stage-1 compound is concluded to.
Thermal Conductivity of Gases. Palm oil RBDNBD 50 - 55. 75-94 Ongokea oil.
Iodine Data Iodine Specific Heat 0214 JgK Sources Occurs on land and in the sea in sodium and potassium compounds. Uses Required in small amounts by humans. A measurement of the thermal conductivity and the specific heat of pure and iodine doped samples of polyacetylene CHx are presented here.
Molar heat capacity of iodine vapour 003112739. Palm olein 56 - 61. Iodomethane also called methyl iodide and commonly abbreviated MeI is the chemical compound with the formula CH3I.
Latent Heat of Fusion of Iodine is 7824 kJmol. It is also produced in vast quantities estimated to be greater than. If the enthalpy of sublimation of iodine is 2 4 c a l g at 2 0 0 o C then the enthalpy of sublimation of iodine at 2 5 0 o C should be.
0 5 5 c a l g respectively. 51 - 54 50 - 55. 50 - 55 Palm oil neutralized and bleached.
Molecular weight of iodine 127g. Water - Specific Heat - Online calculator figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to 360 C 32-700 F -. Iodine is a chemical element with the symbol I and atomic number 53.
Thermal Conductivity Common Liquids. Latent Heat of Fusion of Iodine is 7824 kJmol. In terms of chemical structure it is related to methane by replacement of one hydrogen atom by an atom of iodine.
Iodine value g100 g Oil Fat. 7 Zeilen Iodine Specific Heat. State at 20 C Solid Description Shiny black non-metalic solid.