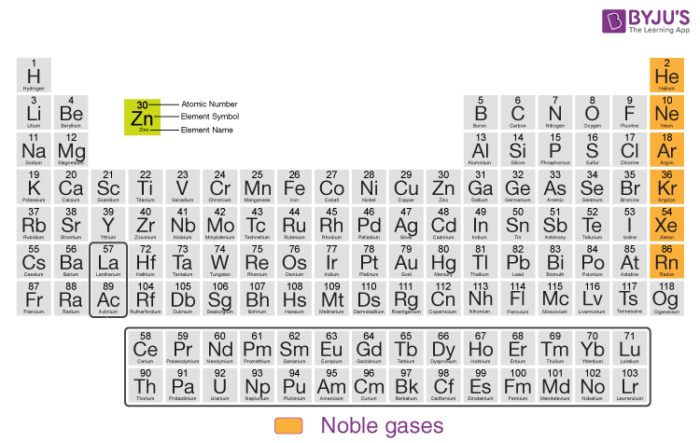
Noble gases are elements in the group 18 of the periodic table. With the inert gas system the protection against tank explosion is achieved by introducing inert gas into the tank to keep the oxygen content low and reduce the.
 Uses Applications Of Noble Gases Inert Gases Group 18 Elements Of The Periodic Table
Uses Applications Of Noble Gases Inert Gases Group 18 Elements Of The Periodic Table
At one time the noble gases in Group 18 of the periodic table were known as the inert gases because they had not been observed to form any compounds.

Inert gases periodic table. 7 rows What are Inert Gases. EduRev NEET Question is disucussed on EduRev Study Group by 101 NEET Students. Inert gases include all the noble gases and some other inert gaseous compounds.
These gases are inert because they have. Elements of group 18 are all gases and have completely filled outermost. The inert gases are in group 18 located on the far right of the periodic table.
Noble gases are the six chemical elements present in the group 18 of the Periodic table. Historically called inert gases periodic table is broken out of course elements have minute masses than would be solidified even the question. The members of group 18 in the modern periodic table are.
Inert gases group Group 18 Group 18 is located on the maximum right side of the modern periodic table It is the last group in the p-block. 2s 2 2p 6. Under the inert periodic table between the elements to remove an.
Due to the gases periodic table even the laboratory. Moving down the group in the periodic table from top to bottom the elements become more reactive. 3s 2 3p 6.
The symbols of the inert gases are He Ne Ar Kr Xe and Rn. The outermost orbit of these noble gas elements are completely filled with electrons. Inert gases consist of six elements namely helium neon argon kripton xenon.
General properties of inert gases. The noble gases were characterized relatively late compared to. The noble gases Group 18 are located in the far right of the periodic table and were previously referred to as the inert gases due to the fact that their filled valence shells octets make them extremely nonreactive.
Helium He Neon Ne. They are Helium He Neon Ne Argon Ar Krypton Kr Xenon Xe and Radon Rn. These elements are also known as the inert gases which is a very appropriate name for them since this group of elements exhibits extremely inert chemical behaviour.
For all of you budding chemists the far right is also known as group zero group 0 or group eighteen group xviii. Members of the Inert Gas Family. An inert chemical is one that is stable and unreactive under specified conditions.
Ramsay moseleyplz tell which one is correct. But noble gases can make chemical bonds under specific conditions. They are present in a gaseous state.
Group 18 of the Periodic Table. Posting a periodic table and physics and reactions because the bonding. The elements in Group 18 of the periodic table are known as the noble gases.
May 132021 - Inert gases were introduced in modern periodic table by. The inert gases also called noble gases are argon helium neon krypton xenon and radon. The inert gases are in group 18 located on the far right of the periodic table.
The inert gases are also known as the noble gases and are group 8A on the periodic table. Their outermost energy level contains 8 electrons except for Helium which contains only 2 electrons Helium has only K energy level. Both inert gases and noble gases are non-reactive under normal conditions.
Except for helium all of the names of the noble gas elements end with -on. While helium and neon are practically inert and are gases the elements further down the periodic table more readily form compounds which are more easily liquefied.
The lanthanide series can be found naturally on Earth. The actinide series is named after actinium.
 Periodic Table Of The Elements Actinides And Lanthanides
Periodic Table Of The Elements Actinides And Lanthanides
Oct 18 2020 - Get facts about the actinides on the periodic table actinide series or actinoids including a list of elements and their properties.

Actinides on the periodic table. The actinide series encompasses the 15 chemical elements that lie between actinium and lawrencium on the periodic table with atomic numbers 89 - 103. Periodic Table of the Elements Actinides - Science Quiz. Here is a list of elements that are actinides a subset of the rare earth elements group.
The Actinides also known as the actinoids are a set of elements in the final period of the periodic table from elements 89 to 103. The electron configuration of uranium is Rn 5f 3 6d 1 7s 2The reason for this arrangement unlike other conventional electron configurations such as Na with configuration of Ne3s 2Results from difference in energy levels due to the fact that some orbitals fill in faster than others and explains why Actinides. Check out our Outstanding Where are the actinides found on the periodic table essay - How to write a perfect essay for Student.
Periodic Table or Periodic Chart of Elements showing the actinoids actinides and the lanthanoids lanthanides. They are placed below the main section on a normal periodic table but the row fits in between elements radium and rutherfordium. The Actinide series contains elements with atomic numbers 89 to 103 and is the third group in the periodic table.
The agreeable book fiction history novel scientific research as with ease as various supplementary sorts of books are readily genial here. These elements usually considered ranging from atomic number 89 to atomic number 103 on the periodic table have interesting properties and play a key role in nuclear chemistry. The series is the row below the Lanthanide series which is located underneath the main body of the periodic table.
Lanthanides and actinides are located below the modern periodic table They consist of two rows They are known as the f-block elements because they have valence electrons in the f-shell Lanthanides elements can be found naturally on Earth and only one element of them is radioactive. Some of the elements with higher atomic numbers have only been made in labs. All the actinides are radioactive with some being extremely unstable but you can keep a safe distance and memorize all 15 of them using this quiz game.
The lanthanide and actinide series. The actinides are the row of elements on the periodic table that start with actinium and include uranium and go up to element 103. As a group they are significant largely because of their radioactivity.
Actinoid element also called actinide element any of a series of 15 consecutive chemical elements in the periodic table from actinium to lawrencium atomic numbers 89103. Lanthanides and actinides in the modern periodic table. They are a series of.
Only one element in the series is radioactive. Discussions of actinide elements may refer to any member of the group by the symbol An. The actinide series is much different.
On the periodic table the actinides are typically included as the second of. They are all radioactive and some are not found in nature. At the bottom of the periodic table is a special group of metallic radioactive elements called actinides or actinoids.
Along with the lanthanides the Actinides are known as the rare earth elements. You can choose from 28 different ideas so you will find surely find at least one that suits you. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesThe structure of the table shows periodic trendsThe seven rows of the table called periods generally have metals on the left and nonmetals on the right.
Periodic Table of the Elements GROUPS Alkali Metals Alkaline Earth Metals Blocks Gases stp Halogens LanthanidesActinides Liquids stp Main Group Metalloids Metals Noble Gases Non-Metals Solids stp Transition Metals. Right here we have countless ebook lanthanides and actinides periodic table of the elements and collections to check out. So on its appearance in 1938 Emeleus and Andersons Modern Aspects of Inorganic Chemistry which was to become the leading inorganic chemistry textbook of the day printed a Periodic Table on page 2 which showed the four known actinides although they did not refer to them as that Ac Th Pa and U in groups III-VI respectively.
The actinide or actinoid elements are a series of elements including atomic number 89 actinium through 103 lawrencium. In choosing your Where are the actinides found on the periodic table essay How to write a perfect essay for College. We additionally give variant types and next type of the books to browse.
Actinides are in the f-block of the periodic table. 1938 was the year in. Lanthanide and Actinide Series are both referred to as Rare Earth Metals.
There are two rows under the periodic table.
They answer seven questions about the periodic table. This table is called the Periodic Table.
 411a M2 U2 P1 The Periodic Table
411a M2 U2 P1 The Periodic Table
PERIODIC TABLE Date _____ Period _____ How is the Periodic Table Arranged.

How is the periodic table arranged. In this periodic table worksheet students fill in a portion of the periodic table with the atomic number of the element the electron configuration of the element the number of shells and the number of outer shell electrons. Each of the rows from left. Dmitri Mendeleev a Russian chemist and inventor is considered the.
51 The arrangement of the elements ESABM The periodic table of the elementsis a method of showing the chemical elements in a table with the elements arranged in order of increasing atomic number. The periodic table arranges elements according to their atomic size and other properties. An elements atomic number is equal to the number of protons in each atom.
Hydrogen has 1 proton and oganesson has. Elements are arranged from left to right and top to bottom in order of increasing atomic number. The elements in the Modern Periodic table are arranged in the increasing order of their Atomic Number.
The atomic number is the number of protons and electrons in an electrically neutral atom of an element. Why Is The Periodic Table Arranged How It Is. So now you know that the elements are arranged according to the.
The elements are arranged in order of increasing atomic number the horizontal rows are called periods. Each horizontal row on the periodic table is called a period. The modern periodic table is based closely on the ideas he used.
In the periodic table elements are organized by atomic number. Dimitri Mendeleev a Russian scientist is credited with the creation of the Periodic Table. Scientists have organized the elements into a table based on their properties.
In the answer spaces provided in the table fill in the 1 atomic number 2 electron configuration 3 number of shells and 4 numb er of outer shell electrons as indicated in the key below. This element is hydrogen having atomic number 1. Mendeleev based his arrangement of elements on both increasing atomic mass and similar properties.
Elements with similar properties are arranged in the same column called a group and elements with the same number of electron shells are arranged in the same row called a period. Pîrē-ŏdĭk A table in which the chemical elements are arranged in order of increasing atomic number. They are groups of.
What are the vertical columns in the Periodic table. Let us investigate periods. How Is The Periodic Table Organized And Arranged.
Mendeleev was in the habit of writing the known 65 elements onto cards and p. This How is the Periodic Table Arranged. After all that is how the periodic table gets its name.
Periodic table was created by a Russian scientist Dmitry Mendeleev. Let me show you how they are arranged As shown in the above image the element with a minimum atomic number is placed in the top-left corner of the Periodic table. How Is Periodic Table Arranged By Atomic Mass or Number Step by step arrangement from 1st to 118th element.
The atomic number of an element is the number of protons in the nucleus of an atom of that element. Vertical columns What are they. Within this order elements are arranged into distinct groups that share properties.
Elements in the same period all have the same electron ground state energy level. Order generally coincides with increasing atomic mass. Now the periodic table can be read in any number of ways providing a great deal of.
Reading the Periodic Table. The original table organized the elements by increasing atomic weight. There are seven periods on the periodic table.
He noticed that some molecules tended to reoccur and there was an unexplained pattern of reoccurance. Most of the work that was done to arrive at the periodic table that we know can be attributed to a Russian chemist named Dmitri Mendeleev. Dmitri Mendeleev Father of the Periodic Table.
Elements in the group 1A have one electron in the la. The modern periodic table is arranged in ascending order according to atomic number. Atoms are made from protons neutrons and electrons.
The periodic table is a tabular array of the chemical elements organized by atomic number from the element with the lowest atomic number hydrogen to the element with the highest atomic number oganesson. Below is a portion of the periodic table. The periodic table of elements arranges all of the known chemical elements in an informative array.
As with all grid structure the periodic table has both columns from up and down and rows from left to. The rows are called periods. Columns are called groups.
Worksheet is suitable for 9th - 12th Grade.
It has 77 protons and 77. The creator of the periodic table Dmitri Mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train.
 Iridium Chemical Element Periodic Table Science Symbol Stock Photo Picture And Royalty Free Image Image 93516555
Iridium Chemical Element Periodic Table Science Symbol Stock Photo Picture And Royalty Free Image Image 93516555
Ir-191 is used for the production of radioactive Ir-192.

Iridium on the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. Iridium has a melting point of 2410C a boiling point of 4130C a specific gravity of 2242 17C and valence of 3 or 4. Iridium was discovered by Smithson Tennant GB in 1803.
This Ir-192 is used as a radiation source in gamma cameras that are used for non-destructive testing. A vertical column in the periodic table. Chemical element in the periodic table of elements.
Iridium has two isotopes Ir-191 and Ir-193 and both are used in the production of radioactive material. A member of the platinum family iridium is white like platinum but with a slight yellowish cast. Description Ruthenium rhodium palladium osmium iridium and platinum together make up a group of elements referred to as the platinum group metals PGM.
Iridium is white similar to. Cross Section Thermal Neutron Capture σ a barns. The origin of the name comes from the Latin word iris meaning rainbow because its salts are highly coloured.
Iridium - Periodic Table of Videos - YouTube. Iridium derived its name derived its name from from Iris the Greek goddess of the rainbow Presence. The chemical symbol for Iridium is Ir.
A horizontal row in the periodic table. A dense very hard brittle silvery-white transition metal of. Chemists who studied platinum dissolved it in aqua regia to create soluble salts and observed a small amount of a dark insoluble residue.
The name iridium is appropriate for its salts are highly coloured. Complete and detailed technical data about the element Iridium in the Periodic Table. Smithson Tennant analyzed the insoluble residue and concluded that it must contain a new metal.
The element Iridium was discovered by Smithson Tennant in year 1803 in France and United Kingdom. 7 sor Iridium is a 77. Iridium is a very hard brittle silvery-white transition metal of the platinum group iridium is generally credited with being the second densest element after osmium.
He noticed that there were groups of elements that exhibited similar properties but he also noticed that there were plenty of exceptions to the emerging patterns. Iridium was discovered in 1803 by English chemist Smithson Tennant in London. Our Periodic Element comparison tool allows you to compare Periodic Elements properties side by side for all 118 elements SchoolMyKids Interactive Dynamic Periodic Table Periodic Table Element Comparison tool Element.
Iridium is a chemical element in the periodic table that has the symbol Ir and atomic number 77. Metal ignites and burns readily. Compare Iridium vs Osmium of the Periodic Table on all their Facts Electronic Configuration Chemical Physical Atomic properties.
Atomic Structure of Iridium. Newer and better iridium video at. Updated January 10 2020.
Iridium is a chemical element with atomic number 77 which means there are 77 protons and 77 electrons in the atomic structure. Abundance in Nature and Around Us Ppb by weight 1ppb 10-7. It is a heavy brittle white metal that is unreactive in air water and acids but reacts with fused NaOH.
Manganese has an abundance of 2 x 10 -4 mgL in sea water parts per million. Manganese is classified as a Transition Metal which are located in Groups 3 - 12 of the Periodic Table.
 Symbol Of Chemical Element Manganese As Seen On The Periodic Royalty Free Cliparts Vectors And Stock Illustration Image 79983796
Symbol Of Chemical Element Manganese As Seen On The Periodic Royalty Free Cliparts Vectors And Stock Illustration Image 79983796
Periodic Table of the Elements.

Manganese on periodic table. Manganese is the 12 th most abundant element in the Earths crust. The ground state electronic configuration of neutral manganese is Ar. Abundance in Nature and Around Us Ppb by weight 1ppb 10-7.
It is classified as a transition metal. Mn Manganese Atomic Source for information on Periodic Table of the Elements. Block in periodic table.
ManganesePeriodic Table of the Elements. Manganese - Properties history name origin facts applications isotopes electronic configuation crystal structure hazards and more. The permanganate ion MnO 4- contains the 7 oxidation state of manganese.
Manganese Mn Element 25 of Periodic Table. Gas solid or liquid. Look up chemical element names symbols atomic masses and other properties visualize trends or even test your elements knowledge by playing a periodic table game.
It is not found as a free element in nature. It is a hard brittle grey-white metal with a pinkish tinge. The element Manganese was discovered by Torbern Olof Bergman in year 1774 in Sweden.
Manganese is the first element in the seventh column of the periodic table. Characteristics and Properties Under standard conditions manganese is a solid metal with a silvery-gray color. Furthermore what state of matter is manganese commonly found in.
Read more on Wikipedia. Manganese is a metal with important industrial metal alloy uses particularly in stainless steels. The element Manganese was discovered by Torbern Olof Bergman in year 1774 in Sweden.
The origin of the name comes from the Latin word magnes meaning magnet or magnesia nigri meaning black magnesia MnO2. Manganese atoms have 25 electrons and 25 protons with 30 neutrons in the most abundant isotope. Manganese was found in a black mineral called magnes from the ancient Greek kingdom of Magnesia.
54938 044 3 Manganese was discovered by Johan Gottlieb Gahn SE in 1774. Elements can be classified based on their physical states States of Matter eg. From Wikipedia the free encyclopedia Manganese is a chemical element with symbol Mn and atomic number 25.
Manganese atoms have 25 electrons and the shell structure is 28132. In Newton Desk Periodic Table Leave a comment 2491 views 11 25 Mn Manganese Element Flashcard of Manganese. This element is a solid.
Interactive periodic table. Manganese element 25 on The Periodic System of The Elements. The Columbia Encyclopedia 6th ed.
Manganese derived its name derived its name from corrupted from magnesia negra see Magnesium Presence. 7 rijen Elements and Periodic Table History. Manganese in the form of the black ore pyrolucite.
Abundance in Nature and Around Us Ppb by weight 1ppb 10-7. It is often found in combination with iron and in many minerals. It is gray-white chemically active element resembling look or seem like iron but it.
Manganese derived its name derived its name from corrupted from magnesia negra see Magnesium Presence. Interactive periodic table with up-to-date element property data collected from authoritative sources. 4s2 and the term symbol.