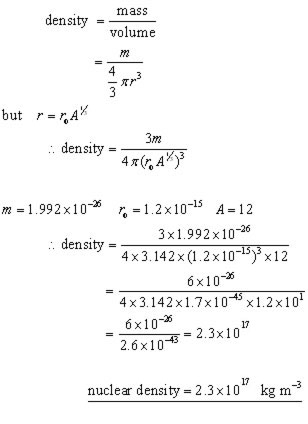The volume V of the nucleus is therefore proportional to the number of nucleons A expressed by 1025 V 4 3 π r 3 k A where r is the radius of a nucleus. Nuclear density is independent of mass number of type of elements.
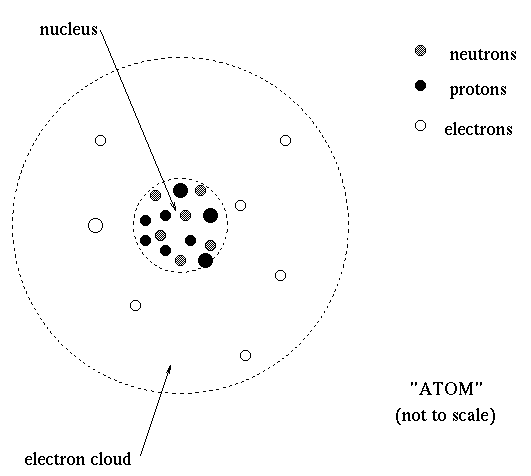 Density The Density Measures How Compact An Object Is That Is How Much Mass It Contains Per Unit Volume To Understand Why The Density Tells Us Something About The Chemical Composition Of An Object Consider The Structure Of An Atom An Atom Is
Density The Density Measures How Compact An Object Is That Is How Much Mass It Contains Per Unit Volume To Understand Why The Density Tells Us Something About The Chemical Composition Of An Object Consider The Structure Of An Atom An Atom Is
The nuclear density for a typical nucleus can be approximately calculated from the size of the nucleus and from its mass.

Density of atomic nucleus. 4 a 5 points An atomic nucleus can be crudely modeled as a gas of nucleons with a number density of 018 fm where 1fm10-15m. It is the ratio of mass per unit volume inside the nucleus. Thus the density of nuclear material is more than 210 14 times greater than that of water.
The usual definition of nuclear density gives for its density. Find the atomic mass of the element silver. As the atomic nucleus carries the maximum mass of an atom and atomic nucleus is small when compared to the entire atom the nuclear density is very high.
Imagine a cube that is 1 mm on a side. This video shows the simple way to calculate the density of nucleus. We can roughly approximate the nucleus as a sphere and thus we can calculate its density where 166 10 27 kg is the mass of the nucleon.
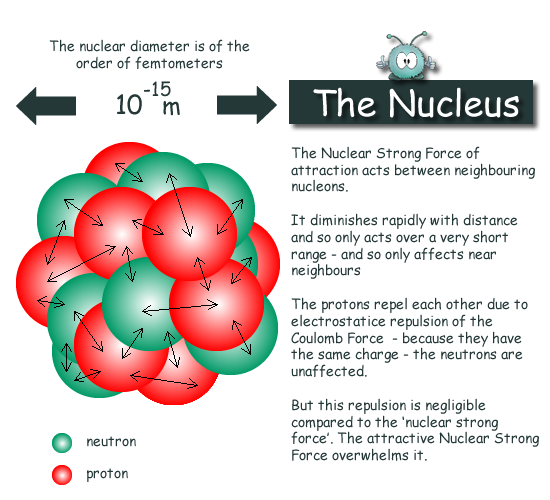
These consist of quarks and gluons which are. It is an immense density. The density of the nucleus of an atom is the nuclear density.
The usual definition of nuclear density gives for its density. Find the atomic mass of the element silver. It is about 23 x 1017 kgm3.
Nuclear densities such as found here are about 2 10 14 times greater than that of water which has a density of only 10 3 kgm 3. The probability density is greatest at r 0 at the nucleus and decreases steadily with increasing distance. Thus the nuclear density is about 200000 tonnesmm 3 and is independent of A.
You use the formula for density and the published radii to calculate the densities. I will only provide a partial working from the example since what follows is irrelevant to my confusion. The simplest model of the nucleus is a densely packed sphere of nucleons.
This video shows how to calculate the radius and density of Radon-222. Thus the density of nuclear material is more than 210 14 times greater than that of water. ρ m v m p A 4 3 π R 3.
Thus the density of nuclear material is more than 210 14 times greater than that of water. Compute the density of a typical nucleus and find the resultant mass if we could manufacture a nucleus with a radius of 1cm. The nucleus carries over 99 of the atomic mass and comprises protons and neutrons.
ρ nucleus m V 238 x 166 x 10 -27 173 x 10 -42 23 x 1017kgm3. ρ nucleus m V 238 x 166 x 10 -27 173 x 10 -42 23 x 1017kgm3. It is an immense density.
At very large values of r the electron probability density is very small but not zero. The values are 670 1014kgdm3 and 27kgdm3. The video also shows how to calc.
It is an immense density. ρ nucleus m V 238 x 166 x 10 -27 173 x 10 -42 23 x 1017kgm3. Atoms have a tiny nucleus and an electron shell some 100000 times larger in diameter than the nucleus itself.
Whereas has atomic mass 108904754 gmol with an abundance of. It is consistent with earlier discussions we have had about the nucleus being very small and containing nearly all of the mass of the atom. The nucleus has atomic mass 106905095 gmol with an abundance of.
We use the assumption that the nucleus is a sphere. Nuclear density 2 x 10 17 kgm 3 Density of solid gold 1932 gmcm 3 Atomic mass 19697 amu 1 mole 19697 grams 1 amu 166 x 10-27 kg Avogadros number 602 x 10 23 atomsmole Calculated atomic radius 13 x 10-10 m Calculated nuclear radius 73 x 10-15 m. Because nucleons come in two different types protons and neutrons each with two possible spin orientations each energy level in the nucleus can hold four distinguishable nucleons.
The usual definition of nuclear density gives for its density. The 1s orbital is spherically symmetrical so the probability of finding a 1s electron at any given point depends only on its distance from the nucleus.
 Is The Density Of The Nucleus Of An Atom Of All The Elements The Same Quora
Is The Density Of The Nucleus Of An Atom Of All The Elements The Same Quora
 Physics Nuclear Physics 4 Of 22 Density Of A Nucleus Youtube
Physics Nuclear Physics 4 Of 22 Density Of A Nucleus Youtube
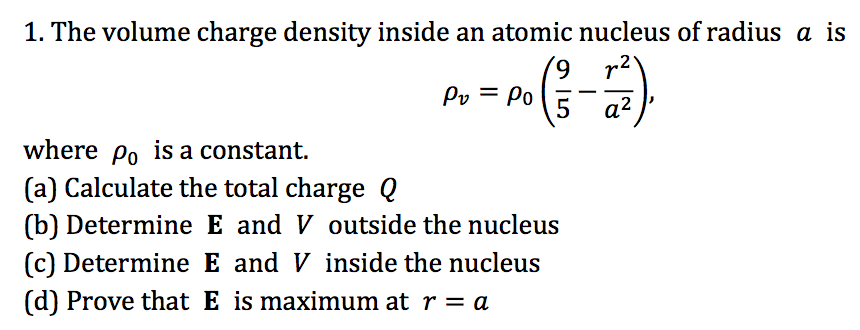 Solved The Volume Charge Density Inside An Atomic Nucleus Chegg Com
Solved The Volume Charge Density Inside An Atomic Nucleus Chegg Com
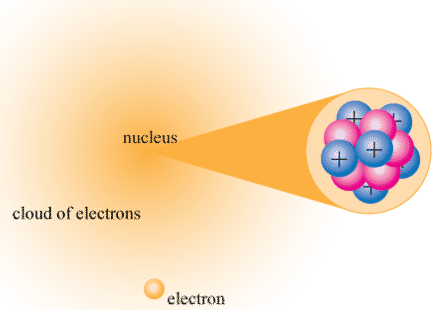 4 14 Atomic Nucleus Chemistry Libretexts
4 14 Atomic Nucleus Chemistry Libretexts
 Nucleus Of An Atom Radius And Density Youtube
Nucleus Of An Atom Radius And Density Youtube
 Volume Of An Atom And Nucleus Nuclear Power
Volume Of An Atom And Nucleus Nuclear Power