To know more differences download BYJUS the learning app. In this case you use the atomic mass in calculations rather than the atomic weight.
What Is The Difference Between Atomic Mass And Atomic Weight Quora
Atomic mass vs Atomic number.
Atomic mass vs atomic weight. If you find an element that exists as only one isotope then the atomic mass and the atomic weight will be the same. If playback doesnt begin shortly try restarting your device. Atoms of the same element with varying atomic masses.
When it comes to differentiating atomic mass versus atomic weight much of the confusion tends to come from the fact that atomic weight is measured in amu atomic mass units instead of being measured in newtons. One is the average weight of an element and the other is the total number of nucleons in the atoms nucleus. In this section carefully compare.
Atomic mass is the weighted average mass of an atom of an element based on the relative natural abundance of that elements isotopes. Although this may be confusing there is a simple explanation. Definition of Atomic Mass Atomic mass is defined as the number of protons and neutrons contained in an individual unit of a substance.
Due to different numbers of neutrons. A B and C. Atomic mass is used to show different isotopes of the same element.
Can Atomic Mass and Atomic Weight Ever Be the Same. Equals MASS number which is the sum of protons and neutrons. The difference is that one is a count of the protons in an elements nucleus while the other is a count of both the protons and neutrons.
It is atomic mass. Roughly equal to the atomic mass. Atomic mass and atomic weight may equal each other whenever you are working with a single isotope of an element too.
Atomic mass also atomic weight is the total weight of an element. Element Q consists of three different isotopes. The key difference between atomic mass and molecular weight is that the atomic mass is the mass of a single atom whereas the molecular weight is the sum of the weights of the atoms in the molecule.
While the atomic weight is a constant for a given element and is reported in the Periodic Table the atomic mass or mass number varies from one isotope to another. To understand the importance well take a look at the standard definitions of mass. What is the difference between atomic mass and atomic number.
See the case of chlorine where atomic weight and standard atomic weight are about 3545. Isotope A has an atomic mass of 40 amu and accounts for 60 of naturally occurring Q. In other places in the text the author refers to the average atomic mass as.
Atomic mass and mass number which are essentially synonymous and atomic weight. Atomic Weight vs. Atomic mass is the mass of an atom whereas atomic weight is the weighted average of the naturally occurring isotopes.
Also represents the mass of one mole of the element in grams. The major difference between atomic mass and atomic weight is atomic mass is a whole number value whereas atomic weight may or may not be a whole number value. Whats the Difference between Mass Number and Atomic Weight.
Atomic mass is also known as atomic weight. Isotopes B has an atomic mass of 44 amu and accounts for 25 of Q. October 25 2011 Posted by Madhu.
Most people use the terms atomic mass and atomic weight interchangeably. Key Differences between Atomic Mass and Atomic Number Atomic number is represented by Z whereas Atomic mass is represented by A. What is properly called the atomic weight is referred to as the average atomic mass and is given the unit amu Thus the atomic weight of copper is listed as 6355 amu.
Atomic mass does not define the type of element whereas Atomic number defines the type of element. The WEIGHTed average of the different isotopes of an atom. So again the mnemonic for memorizing the difference between atomic mass and atomic weight is.
There are a few different terms used by chemists to describe the heaviness of an element. One difference between atomic mass and molar mass to take note of is that the latter refers to the weight of a mole of a substance while the latter refers to the weight of the molecules of a substance. An atom has weight.
For non-mononuclidic elements that have more than one common isotope the numerical difference in relative atomic mass atomic weight from even the most common relative isotopic mass can be half a mass unit or more eg. The key difference between atomic weight and atomic mass is that atomic weight is the average weight of an element with respect to all its isotopes and their relative abundances but atomic mass is the mass of a single atom. Videos you watch may be.
Atoms are the building blocks of all matter. It is found by adding together the number of protons and neutrons in the.
Difference Between Atomic Mass And Atomic Weight Atomic Mass Vs Atomic Weight
 Mcat Mnemonics Atomic Mass Vs Atomic Weight Prospective Doctor
Mcat Mnemonics Atomic Mass Vs Atomic Weight Prospective Doctor
 What S The Difference Between Mass Number And Atomic Weight Youtube
What S The Difference Between Mass Number And Atomic Weight Youtube
 Atomic Weight And Average Atomic Mass Chemistry Tutorial Youtube
Atomic Weight And Average Atomic Mass Chemistry Tutorial Youtube
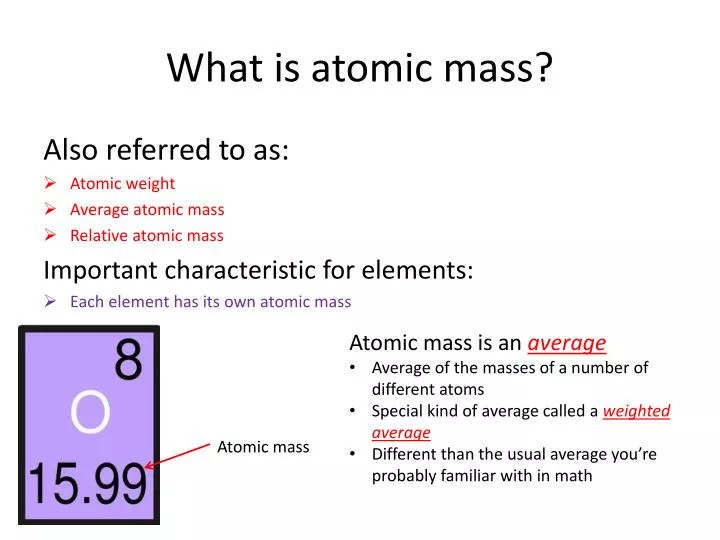
 Ppt What Is Atomic Mass Powerpoint Presentation Free Download Id 2369294
Ppt What Is Atomic Mass Powerpoint Presentation Free Download Id 2369294
 Atomic Mass Vs Atomic Number What Is The Difference Diffzi
Atomic Mass Vs Atomic Number What Is The Difference Diffzi
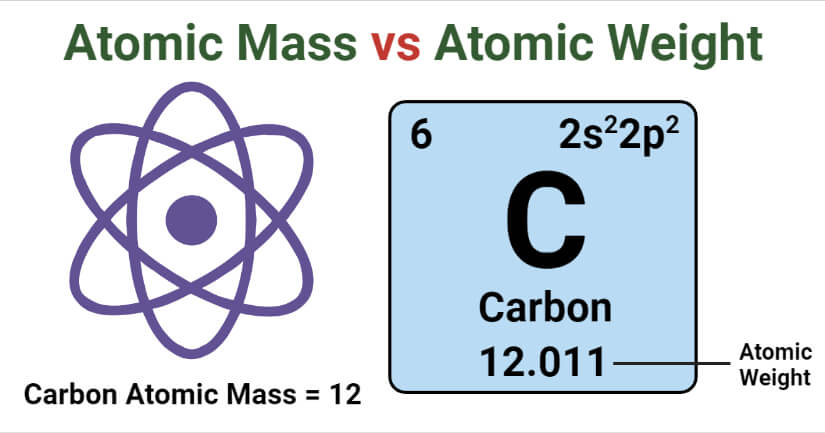 Atomic Mass Vs Atomic Weight Definition 7 Major Differences
Atomic Mass Vs Atomic Weight Definition 7 Major Differences
 Difference Between Atomic Weight And Atomic Mass Compare The Difference Between Similar Terms
Difference Between Atomic Weight And Atomic Mass Compare The Difference Between Similar Terms
:max_bytes(150000):strip_icc()/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)